If you’re a parent, you might have noticed toddler “milk” while browsing the formula aisle. The powdered drink, aimed at children between 1 and 3, often pledges benefits like “improved brain development” or “improved immune function.”
But you may not know that these products are largely unregulated and make claims that are not supported by science, according to studies. For this reason, among others, public health authorities around the world have long sought to police such advertising. Yet despite these efforts, toddler milk has grown to become a $20 billion global business.
As ProPublica reported recently, the U.S. government has played a key role in that growth.
We found federal officials have worked for decades with the multibillion-dollar baby formula industry to mount a global campaign to suppress regulations such as marketing bans — often, critics say, at the expense of public health, particularly in developing countries.
Toddler milk, it turns out, is just the latest chapter in this long-running saga.
Below is a list of key questions and answers about baby formula companies’ business overseas and how the U.S. government has supported those corporate interests.
1. What is baby formula and when is it typically used?
According to the Federal Food, Drug, and Cosmetic Act, infant formula is a special food suitable to serve as a complete or partial substitute for breastmilk. It’s used for babies under age 1.
2. Why is baby formula advertising regulated?
Formula is one of only two products in the world for which there are international recommendations that countries prohibit their marketing. The other is tobacco.
People in the U.S. today may find that shocking, since formula is a regular part of life for many parents. But more than four decades ago, concerns about unregulated advertising of formula surfaced after health advocates found that companies such as Nestle had targeted developing nations in places like Africa in hopes of increasing formula sales. Thousands of babies were growing ill and dying because these populations had neither the clean water they needed to mix the formula safely nor the resources to buy enough of the expensive product.
In response, the World Health Organization’s member nations established an international code advising countries to prohibit the marketing of infant formula.
3. Why do medical professionals generally agree that breastfeeding babies is preferable to formula?
The WHO and UNICEF recommend that babies breastfeed exclusively for six months and continue through their second birthday and beyond as other foods are introduced. The benefits are well-documented. Studies have found fewer infant deaths and infections among breastfed children and fewer incidences of long-term conditions like diabetes and obesity.
The formula industry acknowledges the benefits. “Breast milk offers a child the best nutritional start in life,” a spokesperson for formula maker Danone said. But “if parents cannot or choose not to breastfeed their baby, formulas are recognized by leading medical societies as the only safe and nutritionally adequate alternative during a baby’s first year.”
Public health advocates, however, worry that the industry’s aggressive advertising — which often includes steep discounts and free samples — will derail a critical cycle for those who intend to breastfeed. Regularly giving your baby formula can cause your breast milk supply to drop, research shows, making your child more reliant on formula.
“The evidence is strong,” a report from the WHO and UNICEF explains. “Formula milk marketing, not the product itself, disrupts informed decision-making and undermines breastfeeding and child health.”

Credit:
June Watsamon Tri-yasakda, special to ProPublica
4. What is toddler formula and what are the concerns about it?
So-called toddler formula, also known as growing-up milk, typically targets children between the ages of 1 and 3 — a time when many parents begin giving their children cow’s milk and more regular foods. Toddler milk often contains nutritional supplements like DHA, an omega-3 fatty acid, and promises benefits for brain and eye health. In Thailand, we even found a brand called “Hi-Q1.”
Health authorities, however, say these claims are dubious. In fact, last fall, the American Academy of Pediatrics warned that toddler milks are “misleadingly promoted as a necessary part of a healthy child’s diet.” The drinks are worse than infant formula for babies under 1 year and do not offer any benefits over cheaper cow’s milk for most children older than 1, according to Dr. George Fuchs III, a lead author of the organization’s report.
Nutrition experts also caution about the hefty doses of sweeteners and sodium in some brands.
The industry defends toddler drinks. They “can contribute to nutritional intake and potentially fill nutrition gaps for children 12 months and older,” according to the Infant Nutrition Council of America, a formula industry trade group.
5. Is toddler formula regulated in the U.S.?
Unlike baby formula, which must meet certain nutritional requirements, toddler formula is not regulated by the Food and Drug Administration in the U.S.
6. What role does the U.S. government play in American companies’ efforts to market baby formula overseas?
Our reporting examined the industry’s interactions with a number of federal agencies. A key one was the Office of the United States Trade Representative, which advises the president on trade policy and seeks to promote American business interests. Records show that USTR staff were in regular contact with formula makers and their industry groups through meetings, calls and position papers. Trade officials then mirrored those positions in communications with other countries or in international forums like the World Trade Organization, documents show.
In many places, the U.S. efforts appeared to succeed. Hong Kong, Indonesia and Thailand, among others, watered down or put on hold regulations aimed at restricting formula advertising after U.S. objections.
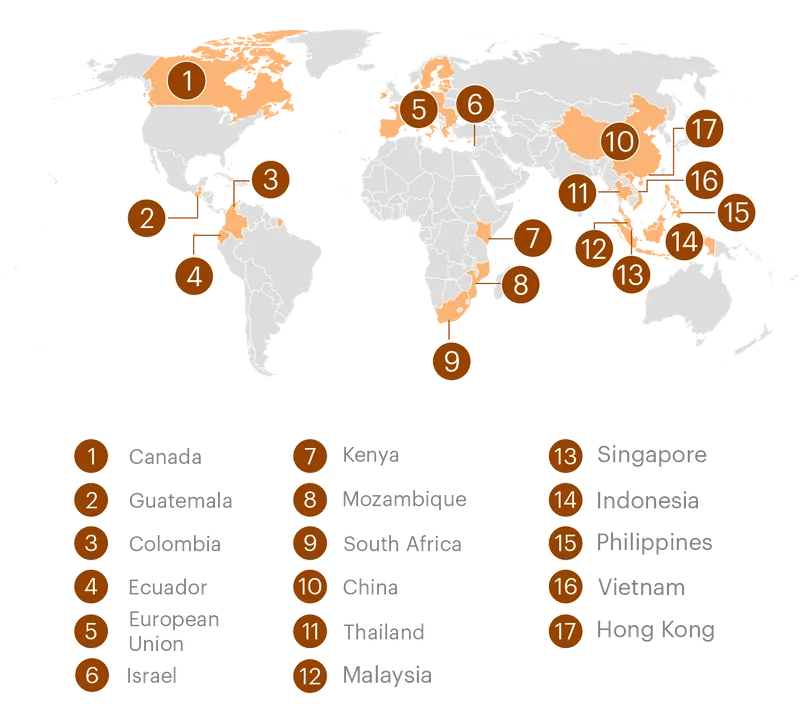
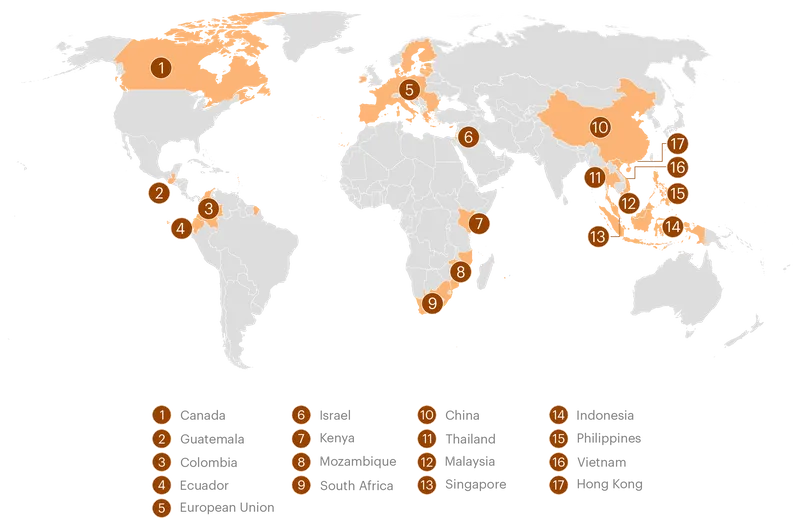
The U.S. Has Waged a Global Campaign Against Formula Regulation
U.S. agencies have intervened in at least 17 jurisdictions over the last several decades on behalf of the formula industry, often to oppose measures that would restrict formula marketing or require additional safety precautions.
Credit:
Lucas Waldron/ProPublica
7. Why has the U.S. government worked to reduce regulations on baby and toddler formulas?
The U.S. is a significant exporter of formula, and the industry has spent considerable resources to protect its financial interests abroad. Its lobbying activity related to foreign health policy ramped up beginning in 2016, as World Health Organization nations considered a resolution aimed at ending the promotion of toddler formula.
That year, the Infant Nutrition Council of America lobbied the USTR and at least four other departments, as well as the Senate and the House, regarding the WHO effort. Leaders in both parties took notice. House Speaker Paul Ryan even called President Barack Obama about the issue, according to records obtained by ProPublica.
Trade officials’ concerns have often reflected those of the industry itself. In one case, they said in a draft letter that proposed rules “would result in significant commercial loss for U.S. companies.” In another case, they worried that a marketing proposal would have a regulatory “spillover” effect in Southeast Asia, one of the industry’s top markets.
The USTR declined to comment on specific cases but said more generally that, under President Joe Biden, the trade office has emphasized respecting the role of foreign governments in deciding the appropriate regulatory approach to infant formula.
8. How is the marketing of baby formula regulated in the United States and abroad, if at all?
In 1981, WHO member nations adopted the International Code of Marketing of Breast-Milk Substitutes, which aimed to curb the worldwide promotion of products that could replace breast milk. The U.S. was the only nation to oppose it.
Since then, at least 144 countries have sought to codify the voluntary restrictions. Such laws often restrict formula marketing in stores, hospitals and elsewhere. Despite poor enforcement in many countries, the laws have had measurable benefits. Countries that have adopted marketing bans have seen their breastfeeding rates rise, studies show, and more breastfeeding is in turn linked to lower infant mortality. It also reduces mothers’ risk of certain cancers.
9. Why are formula companies so focused on developing nations?
Developing economies represent big business for the formula industry. One academic study found that low- and middle-income countries accounted for more than 90% of the roughly $19 billion in toddler milk sales in 2022.
As incomes have risen in those countries, formula makers saw an opportunity. “In most countries, breastfeeding is incompatible with women participating fully in the workforce,” Kasper Jakobsen, CEO of the formula company Mead Johnson, said in a 2013 earnings call. “As women participate in the workforce, that creates a rapid increase in the number of dual-income families that can afford more expensive, premium nutrition products.”
Today, Southeast Asia is more important to the formula industry than the U.S. and European markets combined.
Infant Formula Looks Nearly Identical to Toddler Milk on a Grocery Store’s Shelves in Bangkok
Thailand’s Milk Code restricts the advertisement of infant formula, but marketing of toddler milk is generally allowed.

10. How has formula marketing affected public health in countries such as Thailand?
Formula marketing can impact a country’s breastfeeding rates and, in turn, its children’s health, since breastfeeding carries benefits such as fewer infant deaths and infections.
Thai officials made a similar argument when they sought to restrict the promotion of infant and toddler formula in 2016. They blamed such advertising, in part, for the nation’s breastfeeding rate, which was among the lowest in the world. Some Thai pediatric authorities also say formula products play a big role in the country’s rising obesity rates because they’re so easy to drink.
The share of Thailand’s babies who are exclusively breastfed for six months has rebounded somewhat but has a long way to go to meet the WHO’s target of 50% by 2025.
11. Is the United States alone in promoting baby formula overseas, or do other countries do the same?
Other dairy and formula-producing countries also promote their products abroad and, at the World Trade Organization, countries such as Australia and New Zealand sometimes join the U.S. in objecting to formula regulations. But the U.S. brings outsized economic and political clout to the debate. “The U.S. is highly influential,” said Dr. Robert Boyle, of the Imperial College London, who has researched international formula use.
12. Is the USTR’s lobbying on behalf of the baby formula industry any different than what it does to promote other U.S.-based companies?
According to the USTR website, part of the office’s job is to work for the “expansion of market access for American goods and services.”
In formulating its official positions on issues, the USTR says it consults with various federal agencies, including those focused on health. But our reporting shows that these debates can be contentious, with trade concerns often trumping public health. In 2016, for example, USTR officials repeatedly questioned well-established science as they sought to water down a WHO resolution that aimed to restrict formula marketing and increase breastfeeding.
The USTR declined to comment on this incident, but it issued a statement acknowledging the office’s “formerly standard view that too often deemed legitimate regulatory initiatives as trade barriers.”
13. The U.S. government has taken pro-industry stances in the Obama and Trump administrations, and it continues to do so in the Biden administration. Is there any reason to believe this might change?
It’s unclear. A USTR spokesperson told us that under Biden, the trade office has been committed “to making sure our trade policy works for people — not blindly advancing the will of corporations.”
But our investigation found several examples of the trade office under Biden handling formula regulation like it did in past administrations. The USTR did not respond to our questions about these cases.